
JCI | TDO2+ myofibroblasts mediate immune suppression in malignant transformation of squamous cell carcinoma
On August 16, 2022, the Fan Bai Group from Biomedical Pioneering Innovation Center (BIOPIC) and Beijing Advanced Innovation Center for Genomics (ICG) in Peking University, collaborating with Zhi Wang group at Hospital of Stomatology, Sun Yat-Sen University, published an article titled TDO2+ myofibroblasts mediate immune suppression in malignant transformation of squamous cell carcinoma in The Journal of Clinical Investigation . This study identified a subset of myofibroblasts expressing tryptophan 2,3-dioxygenase (TDO2), which played a critical role in T cell sequestration and suppression. This study provides a multistep transcriptomic landscape of OSCC and demonstrates that TDO2+ myofibroblasts are potential targets for immunotherapy.

Oral squamous cell carcinoma (OSCC), a malignancy that occurs in the epithelium of the oral cavity, is the predominant type of head and neck squamous cell carcinoma (HNSCC). Oral carcinogenesis is a multistage process that initiates from oral precancerous lesions, of which oral leukoplakia (OLK) is the most common type [1]. A recent cohort study revealed that the malignant transformation rate of OLK is approximately 11.7-23.1% [2]. Interventions need to be delivered during the early stage of OSCC to prevent its progression and achieve a better prognosis for OSCC patients.
With single-cell RNA sequencing (scRNA-seq) and paired single-cell T cell receptor (TCR) sequencing on 13 OSCC samples, 3 OLK samples and 8 adjacent normal samples, the researchers obtained 131,702 high-quality cells, which were partitioned into 10 major clusters (Fig. 1).
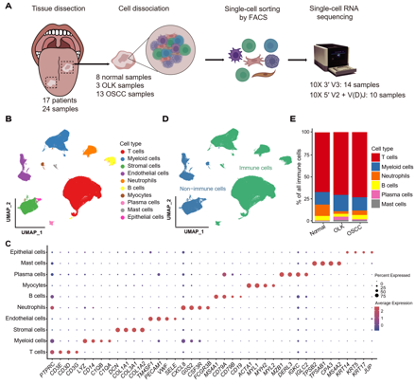
Fig. 1 Single-cell transcriptomic landscape of adjacent normal, OLK and OSCC tissues
The researchers observed that the relative percentages of resting and activated regulatory T cells (Tregs) and exhausted CD4+ T cells showed a gradual increase from normal to OLK to OSCC tissues. Transitory CD4+ T cells with a strong proliferative capacity differentiated into activated Tregs and into exhausted CD4+ T cells. The relative percentages of terminal exhausted CD8+ T cells, precursor exhausted CD8+ T cells and transitory exhausted CD8+ T cells in OSCC tissues were higher than those of their OLK and normal counterparts. And the precursor exhausted and transitory exhausted CD8+ T cells switched into terminal exhausted CD8+ T cells. This study suggests that T cells become functionally impaired early in the premalignant stage of carcinogenesis, after which they transform into an inhibited phenotype (Fig. 2).
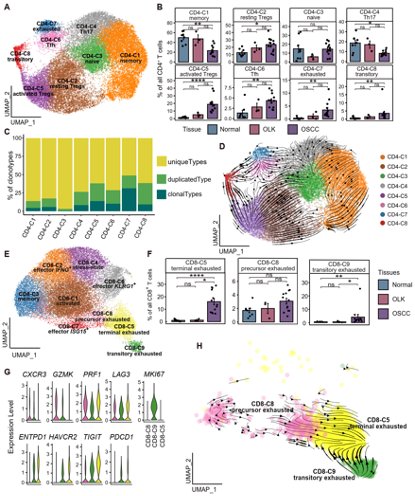
Fig. 2 T cell dysfunction and cell state transitions in OSCC
In stromal cells, the researchers found that 2 subsets of myofibroblasts (MF-C1-TDO2 and MF-C2-ELN), which were nearly absent in normal tissues, slightly more common in OLK, and substantially more abundant in OSCC. The ELN+ subset functions in matrix remodeling, while the TDO2+ subset functions in immune regulation. Immunofluorescence (IF) imaging illustrated that TDO2 was predominantly expressed in α-SMA+ myofibroblasts in OSCC, whereas it was nearly absent in normal tissues (Fig. 3). Multiplex immunohistochemical staining (mIHC) showed that in OSCC samples, myofibroblasts (α-SMA+) were mainly located on the periphery of tumor nests (Pan-CK+) (Fig. 4). High-content cell imaging demonstrated that, over time, TDO2+ myofibroblasts possessed a much stronger T cell chemoattractant function in comparison with that of TDO2- myofibroblasts.

Fig. 3 A subtype of myofibroblasts expressing TDO2 in OSCC
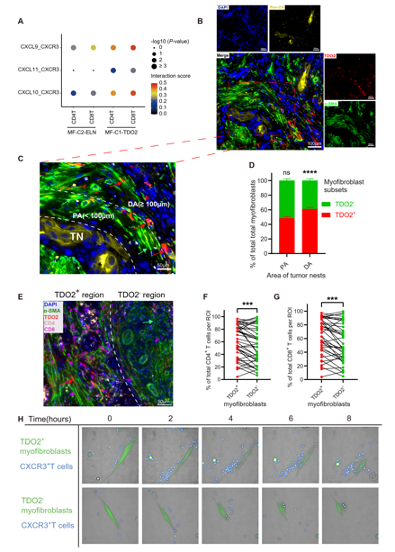
Fig. 4 MF-C1-TDO2 attract T cells and shield tumor cells from T cell attacks
mIHC demonstrated that Foxp3+ CD4+ T cells were significantly more abundant around TDO2+ myofibroblasts than TDO2- myofibroblasts, and found that TDO2+ myofibroblasts and terminal exhausted CD8+ T cells (TIM-3+ PD-1+ CD8+ T cells) tended to be in close proximity with one another (Fig. 5). To explore whether TDO2+ myofibroblasts exert an immunoregulatory effect on T cells after attracting them, the researchers transfected OSCC-derived myofibroblasts with small interfering RNA (siRNA) to knockdown TDO2 expression in vitro and performed the coculture experiment between myofibroblasts and CD4+ or CD8+ T cells. The coculture results showed that the silencing of TDO2 in myofibroblasts resulted in downregulated expression of Foxp3 in CD4+ T cells and PD-1 in CD8+ T cells, while the secretion of granzyme B (GZMB) increased in CD8+ T cells. All of these results suggest that TDO2+ myofibroblasts might mediate T cell suppression, and the suppressive status of T cells was reversed by the addition of the TDO2 inhibitor LM10. Survival analyses reveal TDO2+ myofibroblasts are associated with a worse prognosis for OSCC patients.
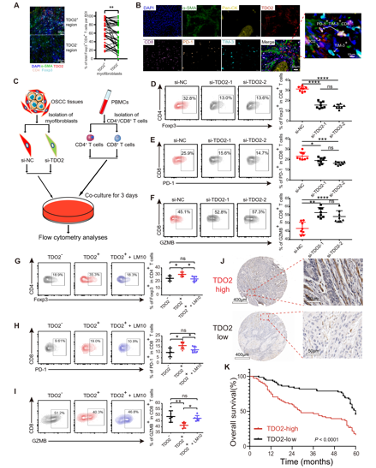
Fig. 5 TDO2+ myofibroblasts mediate T-cell suppression
To examine the effects of TDO2 inhibitors on OSCC in vivo, the researchers established a 4NQO-induced carcinogenesis model in immunocompetent mice and used TDO2 inhibitor LM10 to treat. Results showed that administration of the TDO2 inhibitor significantly suppressed the formation of OSCC in 4NQO-treated mice (Fig. 6). Subcutaneous tumorigenesis models indicate that TDO2 inhibitors can inhibit tumor growth and promote anti-PD-1 efficacy and that the antitumor activities are dependent on T cells (Fig. 7).

Fig. 6 Inhibition of TDO2 prevents the formation of OSCC
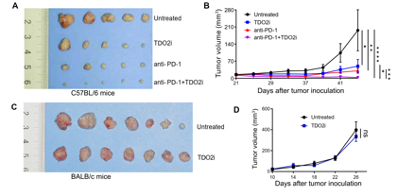
Fig. 7 The combination of TDO2i and anti-PD-1 inhibit tumor progression
This study characterized the dynamic changes in T cells, myeloid cells, neutrophils and stromal cells during the process of oral carcinogenesis. Notably, the researchers discovered a subset of myofibroblasts expressing tryptophan 2,3-dioxygenase (TDO2), which played a critical role in T cell sequestration and suppression. Moreover, the researchers confirmed that the silencing of TDO2 in myofibroblasts and the TDO2 inhibitor LM10 could reverse the inhibitory state of T cells and prevent the progression of OSCC in murine models. Their findings reveal a novel immunosuppressive mechanism by which stromal cells regulate T cells and provide a potential therapeutic target for OSCC.
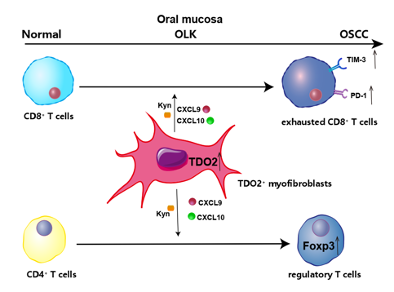
Fig. 8 TDO2+ myofibroblasts mediate immune suppression in malignant transformation
Ph.D. candidate Simeng Hu, Dr. Huanzi Lu, Dr. Wenqiang Xie and Dr. Dikan Wang are the co-first authors of the paper. Prof. Zhi Wang from Hospital of Stomatology, Sun Yat-Sen University and Prof. Fan Bai from Peking University are the co-corresponding authors of the paper. This research was financially supported by the National Science and Technology Major Project (2019YFC1315702) and Guangdong Province Key Research and Development Program (2019B020226002).
Link: https://www.jci.org/articles/view/157649
References:
[1] Yardimci G et al., Precancerous lesions of oral mucosa, World J Clin Cases (2014).
[2] Evren I et al., Annual malignant transformation rate of oral leukoplakia remains consistent: A long-term follow-up study, Oral Oncol (2020).